It’s August again, and it’s time to ready those lesson materials on the classification of matter. Boy, I have learned a lot over the years about what to do and what NOT to do when rolling out the classification of matter!
How the classification of matter is usually taught...
Traditionally, the classification of matter is taught through direct instruction and guided practice.
- Can students identify whether this picture (or formula) represents an atom, compound, or molecule?
- Can students distinguish between homogeneous mixtures and heterogeneous mixtures?
What I've started to realize though...
As a teacher, however, I’ve slowly started to realize something over the years. Diving head first into atoms versus elements, compounds versus mixtures, and colloids versus suspensions… may not be the wisest move at the start of the school year…
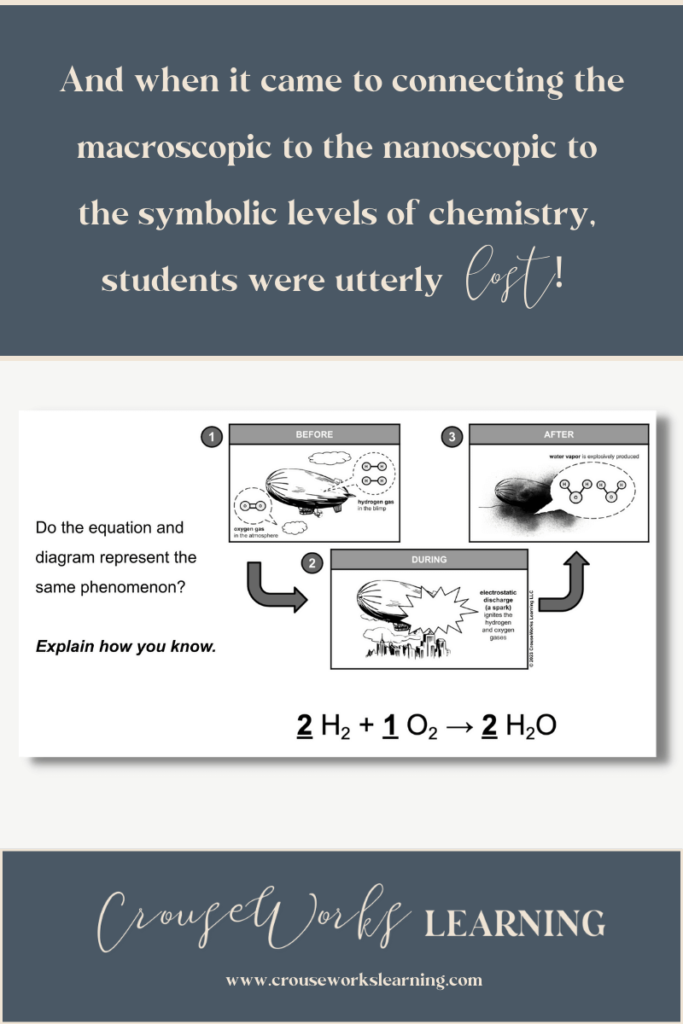
Think about it…for you and me, as chemistry teachers, understanding diagrams like the one you see below is second nature.
But for students, what do they see?… they see a minefield of specific facts they need to memorize- and quickly!
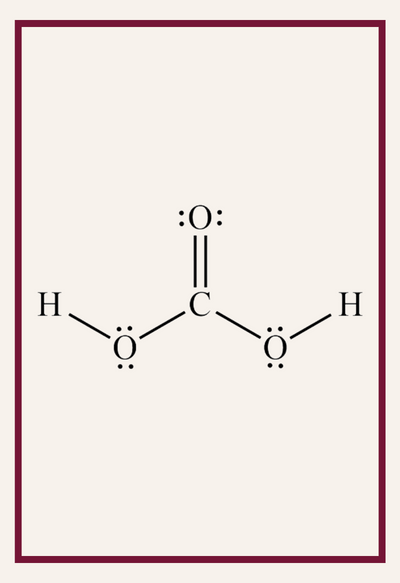
For example, think about all the skills students juggle when they look at this ONE picture…
- Why do the O’s have dots around them?
- Why are there lines between the letters?
- Why do some letters get two lines?
- Is CO the same as Co on the periodic table?
It took me a long time to realize how many skills students juggled when looking at this one diagram. I never appreciated that students genuinely learned a new language when they entered the chemistry classroom. And just like we wouldn’t jump into conjugating verbs in Spanish class during the first week of school, we shouldn’t push students to distinguish between minute details like the Tyndall effect and what is a suspension vs. a solution vs. a colloid- especially if they can barely grasp what atoms are!
How the classification of matter should be taught...
For years, I hammered the hierarchical classification on matter chart… you know, the one that looks like the picture you see below…

And yes, by the end of the unit, students could certainly tell me the difference between a pure substance and a mixture. But when it came to connecting the large-scale (macroscopic) level to the small-scale (nanoscopic) level to the formula (symbolic) level, they were utterly lost!
Think about it, which skill is more important… memorizing a hierarchy of nuanced terms in chemistry or ensuring students understand how atoms, molecules, and chemical equations actually represent real-world, large-scale phenomenon?
In chemistry, it doesn’t matter in the long run if kids cannot recognize molecules versus compounds or solutions versus suspensions. But it does matter if students cannot recognize how different layers of the world (i.e., the macroscopic, nanoscopic, symbolic) connect.
Suppose your students can take a picture like the one you see below and explain the macroscopic (visible), nanoscopic (invisible), and symbolic levels of it. In that case, they have a practical knowledge of chemistry that will carry them much further than simply memorizing pure substances versus mixtures.
For better or worse, though, many of us are under pressure to pass students on district chemistry exams at the end of the semester. And on those exams are questions like “What is a homogeneous mixture vs. a heterogeneous mixture?”
So how do you balance, as a teacher, helping students see the bigger picture of chemistry while still also knowing the details they need to be successful on the semester exam?
Please read below for some helpful strategies to strike this balance!
TIP #1: Make learning the classification of matter hands-on!
As much as I hate to admit it, students don’t care at the end of the day what a pure substance is versus a mixture- no matter how I phrase it or spruce up my slides with great pictures…
However, students WILL care about pure substances versus mixtures when you task them with separating pepper, salt, iron filings, and sand from a homogeneous mixture. And even more so when you only give them a beaker, magnet, and some tweezers to work with. Just as a caveat, I have to tell you… the number of years I have seen students spend entire class periods trying to tweeze out sand from pepper from salt… is just too funny! Sooner or later, though, students’ background knowledge often kicks in. Iron is magnetic. Salt will dissolve in water. Sand is dense and will sink in water. Pepper will float in water.
And what a relief for students! Sand, salt, pepper, and iron are each pure substances with unique properties that allow them to be separated.
This is a step forward in students appreciating why scientists classify matter and how knowing the properties of substances can be helpful!
TIP #2: Teach the classification of matter AFTER you teach students physical and chemical changes and properties
When you teach the classification of matter AFTER physical and chemical changes and properties, students will more easily understand that the properties of pure substances change when they combine to form compounds. Since students are already familiar with the chemical and physical properties, it is not a cognitive lift to imagine that the properties of substances can change when matter changes.
And ultimately, the real reason we care about organizing matter into atoms, compounds, mixtures, etc., is because it tells us about the physical and chemical properties of the substances! I always tell students, “We are NOT learning this because we want to go out into the world and point to things that are atoms versus compounds versus heterogeneous mixtures. We are learning this because knowing what a substance is will tell us a lot about its properties and what we can do with it.”
TIP #3: Connect the classification of matter to real world examples, like air quality
Sometimes, finding tangible, easy-to-understand connections to real-world topics is hard work in chemistry. And the classification of matter is one of those not-so-relatable topics at first glance.
Yes, you can certainly talk about scientists separating pure substances from mixtures when they refine ores during the mining process… but, let’s be honest, most students don’t actually care about that kind of stuff.
So what about, instead, relating your lesson to air pollution? Students are often surprised to learn that air is a mixture. But when they find out that air is not only a mixture but a homogeneous mixture (i.e. it has a uniform composition), it makes more sense why it’s been so difficult for scientists to isolate and extract individual pollutants from the air (compounds like carbon dioxide, nitrous oxide, and sulfur dioxide).
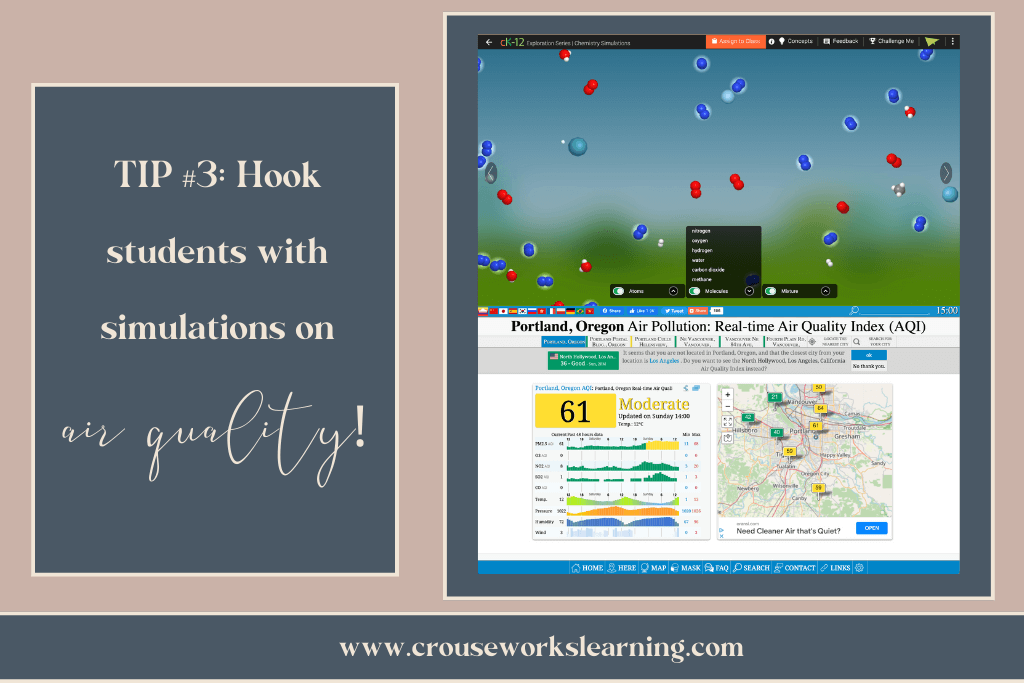
- Click here for an excellent simulation from CK-12 that allows students to see the molecules that make up air. NOTE: You will need to set up a CK-12 account, but it is completely free!
- Click here for another fun website that allows students to see the air quality measurements near their homes.
And if you want to provide students with further background information on the sources and dangers of different air pollutants, check out this simple table I made below.
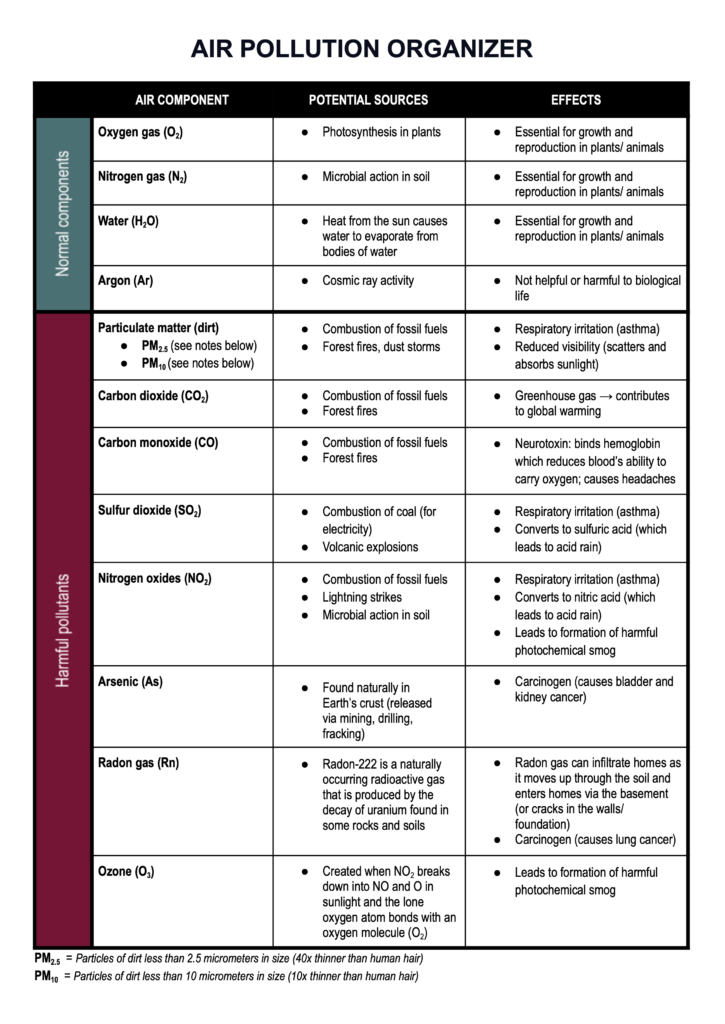
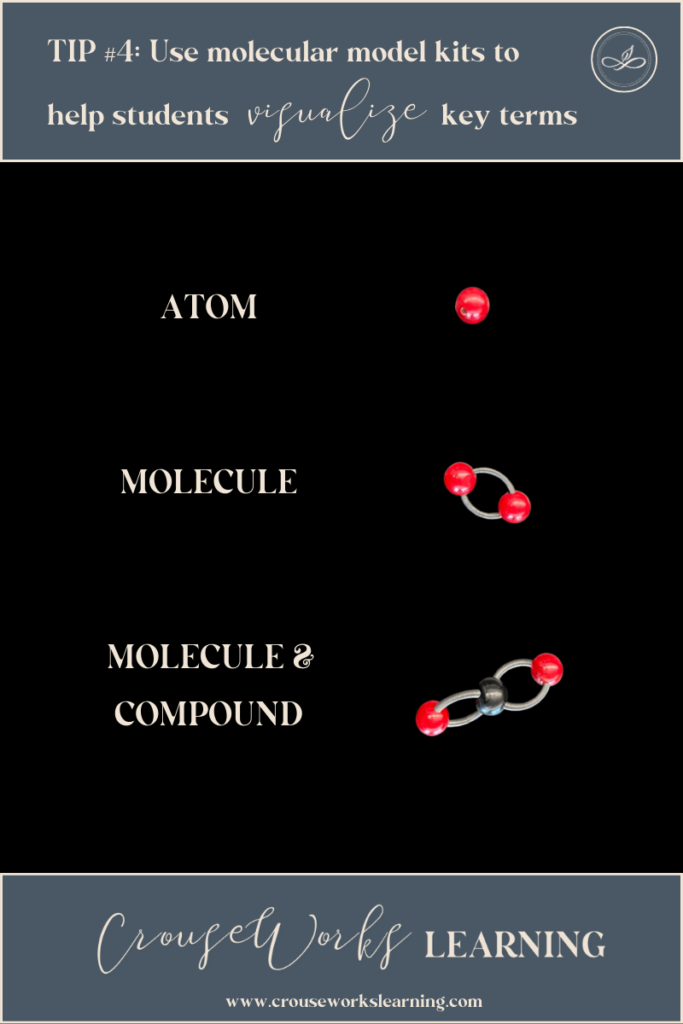
TIP #4: Use molecular model kits to show students how matter is classified
If you have not considered purchasing molecular model kits for your chemistry classroom, I highly recommend it. Molecular model kits provide a great, hands-on visual of our nanoscopic world!
They come in handy not only when you want to distinguish between elements, atoms, molecules, and compounds, but they also come in handy when students need to conceptualize reactants versus products in a reaction.
TIP #5: Connect the classification of matter to scientific modeling
Sometimes it can be tough to squeeze in a lesson on scientific modeling during chemistry. But the classification of matter is made for scientific modeling!
Show students different diagrams of the same molecule and ask them…
- What are the advantages and disadvantages of each model?
- Why would scientists make different models for the same molecule?
- Which model do they prefer looking at?
Students, surprisingly, have a fun time looking at different types of models. They like identifying the different numbers and types of atoms present in molecules. Which is a great way to begin building students’ confidence in chemistry!
To wrap up...
I’ll admit, for years, the classification of matter was something that caused even me, as a teacher, to get lost in the weeds. There’s a lot to sift through…
- What’s relevant for students to know? What’s not relevant?
- What material will my students be tested on for the district final?
- How can I make a topic like this engaging for students?
- How can I help them retain this knowledge better?
I hope this blog post has given you some helpful tips and tricks for your next classification of matter lesson. The classification of matter is a tricky subject, but when it’s done right, it can really hook kids into chemistry at the start of the year!

Excited to try teaching the classification of matter, but wondering how to go about it?
Check out this resource which includes:
- An easy-to-set-up lab
- Student-led inquiry investigation
- Text analysis
- Application problems
Looking for more tips and tricks on how to get students motivated at the start of the school year in chemistry?
Check out this beginning-of-the-year NGSS phenomenon-based unit on wildfires (click here)